Young people to face ‘intrusive and distressing’ tests in NHS puberty blocker trial

Details on the NHS puberty blockers trial have been shared. (Getty/Canva)
Details on the NHS puberty blockers trial have been shared. (Getty/Canva)
Youngsters under the age of 16 will reportedly have to undergo 13½ hours of medical assessments before being able to access trans healthcare under a new trial for puberty blockers.
Details of the NHS-commissioned study of the effectiveness of puberty-blocking medication were announced over the weekend. Researchers from King’s College London said the two-year Pathways trial would analyse the physical, social and emotional wellbeing of about 220 young teenagers.
However, campaigners have described the trial as “intrusive and distressing”.

Commissioned in the wake of the government’s ban on the medication, the £10 million ($13.1 million) trial is the only way to be prescribed puberty blockers on the NHS.
According to a clinical document, young people chosen to take part will have to fill in some 50 forms, including questions on trauma and suicide, and undergo bodily examinations. Half the patients will receive treatment, while the other 110 will have to wait a year to access healthcare.
Emily Simonoff, a professor of child and adolescent psychiatry at the Institute of Psychiatry, Psychology and Neuroscience at King’s College, told Radio 4 that the study was not expecting a “one size fits all finding”, adding: “We are looking at the balance between, possibly, benefits for mental health and quality of life, and any possible risks or harms.”
Health secretary Wes Streeting said the trial was launched in the wake of recommendations from the Cass Report, which has itself faced criticism. In October, a group of Australian paediatricians wrote: “A patient’s goal of achieving optimal quality of life as a trans person requires respect. The Cass Review, lacking expertise and compromised by implicit stigma and misinformation, does not give credible evidence‐based guidance. We are gravely concerned about its impact on the well-being of trans and gender‐diverse people.”
“Dr Cass recommended a ban on prescribing [puberty blockers] and a clinical trial to build that evidence. King’s College London has now launched that trial,” Streeting wrote online.
NHS England’s decision to effectively ban all puberty blocker prescriptions was made four weeks before the Cass Report was published.
‘This is not a clinical trial, it’s cruelty’
Caroline Litman, the mother of trans teen Alice, who took her own life while on an NHS waiting list, criticised the trial, saying: “This is not health care. The old ‘watch and wait’ is how Alice was treated, although this time there might be someone actually watching.
“This is not a clinical trial, it’s cruelty.”
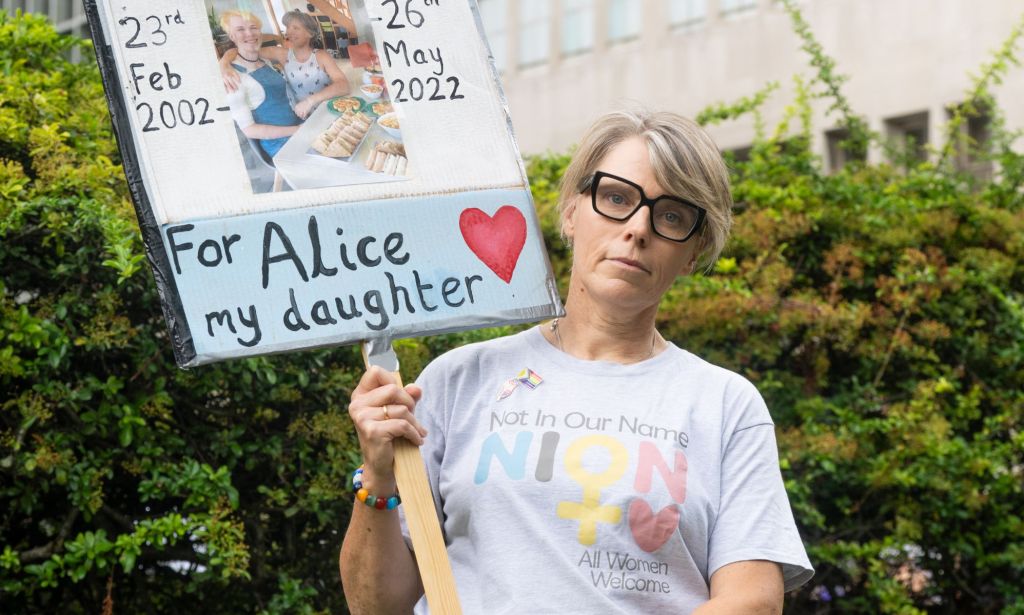
According to recent research, not being able to get prescriptions for puberty blockers in the UK is having “serious adverse effects” on young trans people. Dr Natacha Kennedy, from the University of London, said there was a notable difference between the mental health of transgender teenagers denied treatment compared with those able to access healthcare.
One parent said their child, who had been “well-adjusted”, developed mental-health issues such as depression and anxiety after being denied puberty blockers earlier this year.
“She had looked forward to this for months, then with no warning it was taken away,” they said. “The shock was awful and she could not cope. My child feels despair, notions of suicide, as puberty… and body changes seem so out of control and irreversible.”
One mother and campaigner, Helen, who goes by the name mimmymum online, called the trial a “huge, time-consuming and emotionally draining obstacle course”. It failed to take other barriers to care into consideration, including the more than four-year wait for a first appointment following a referral, and the “barrage” of physical and psychological exams were “intrusive and distressing”, she added.
“It is a system that places unbearable pressure on the very young people it claims to support. Instead of helping them, it risks hurting them.”
“I am surprised that this trial received ethical approval”
Chay Brown, the director of operations at advocacy group TransActual, said: “It breaks my heart when I think of the young people affected. This research is not about the safety of these medications which have been used for this very purpose since 1989. It is the result of an ideological view at the top of the NHS that being trans is a ‘less desirable outcome’.
“This goes against every assurance made previously. I am surprised that this trial received ethical approval. Hard questions will one day be asked of those who have given this trial the fig leaf of ethical approval.”
Share your thoughts! Let us know in the comments below, and remember to keep the conversation respectful.

